The working principle of a supercapacitor
Electrochemical double-layer capacitors (EDLCs) consist of two electrodes that are separated by a separator, soaked with an electrolyte. They store energy by forming double layers at the electrode-electrolyte interfaces. The double-layer formation is an extremely fast process and therefore EDLCs/supercapacitors can be charged/discharged within seconds, making them high-power devices. This physical storage process is usually highly reversible, allowing a long cycle life of over 100 000 cycles. The capacitance and power of a supercapacitor are determined by the interaction of the electrode material with the electrolyte. Activated carbons are the most used electrode material for both electrodes due to their high specific surface area, forming a large interface when in contact with an electrolyte.
The most widely used electrolytes for supercapacitors are based on tetraalkylammonium salts, e.g. tetraethylammonium tetrafluoroborate (TEA-BF4) dissolved in organic solvents, such as acetonitrile. These combinations of salts and solvents enable high ionic conductivities and low viscosities that are highly important for fast double-layer formation and high power.
The operating voltage of a supercapacitor is limited by the electrochemical stability of the applied cell components, in particular of the electrolyte. A high purity of the electrolyte components is essential, since impurities can cause severe degradation, e.g. gas formation, especially at high temperature and elevated operating voltages. Various approaches are currently investigated to increase the voltage of supercapacitors from around 2.8 V today to above 3.0 V and the operation temperature to above 60 °C with the help of novel electrolytes.
Step 1 – Initial state
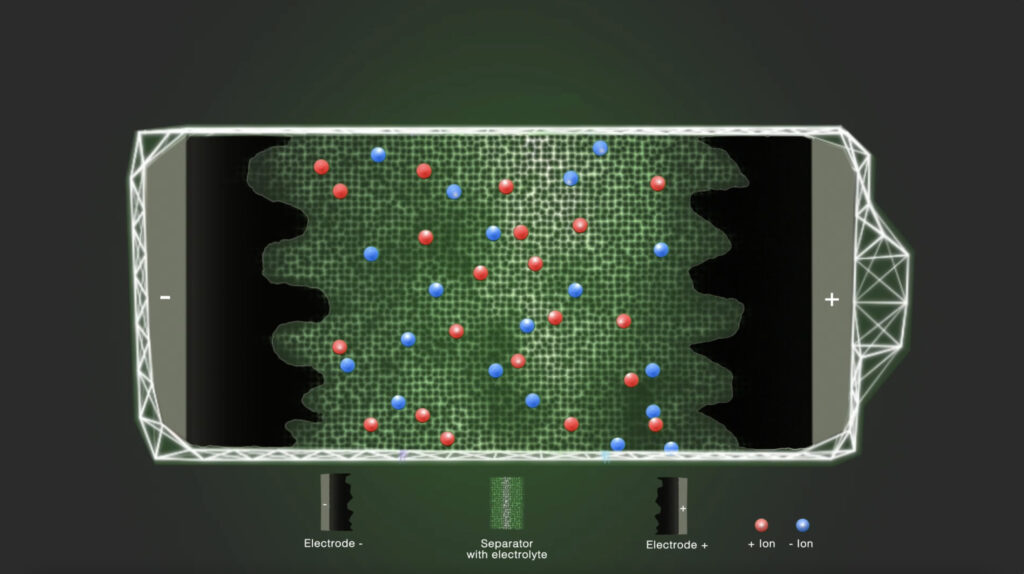
In the discharged state, the positively and negatively charged ions are equally distributed in the electrolyte solvent. The electrolyte ionically connects the electrodes with each other.
Step 2 – Charge
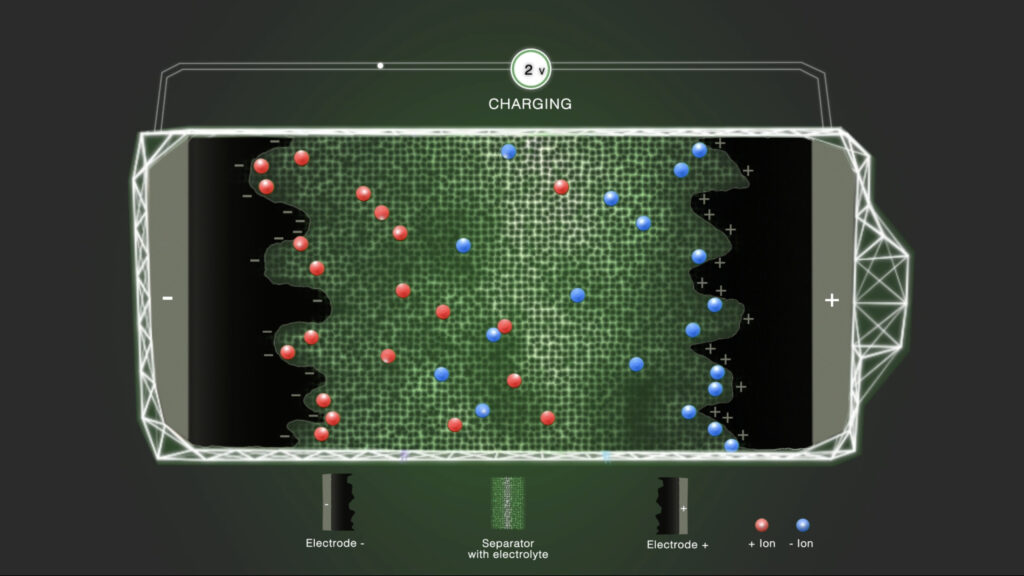
When a voltage is applied, one of the electrodes is positively charged and the other electrode is negatively charged. This causes the ions present in the electrolyte to migrate and diffuse to the respective electrode with the opposite charge. The positively charged ions are attracted to the negative electrode and the negatively charged ions to the positive electrode. The by Coulombic forces attracted ions are loosely associated with the electrodes, leading to the formation of an electrostatic double layer. In contrast to batteries, only electrostatic effects contribute to the storage of electrical energy in supercapacitors.
Step 3 – Double layer
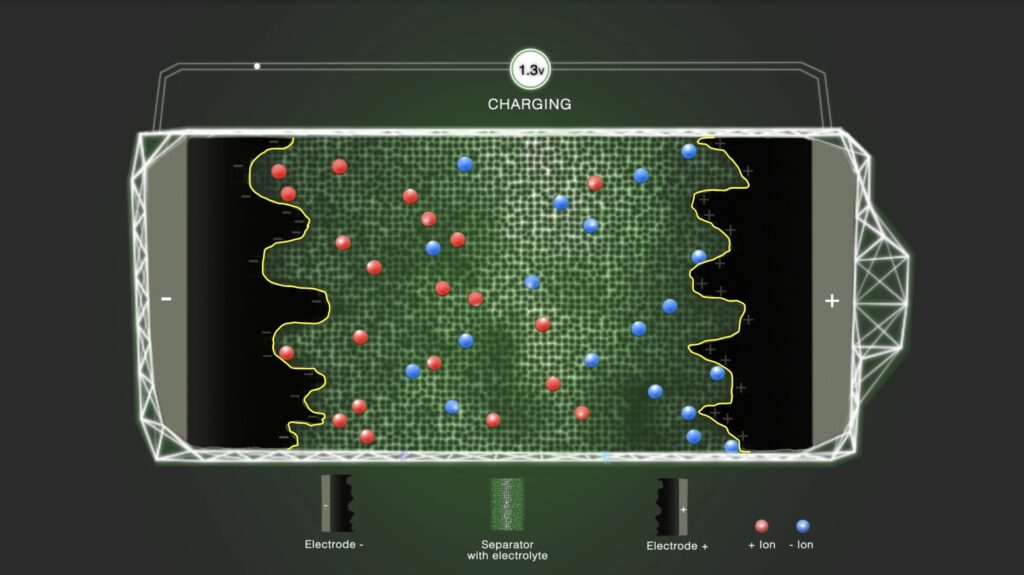
By charging the capacitor, ions in the vicinity of the electrodes are accumulated stepwise at the boundary between the electrode and the electrolyte. The double-layer formation takes place at the electrode/electrolyte interface; therefore, the active materials and the electrolyte must be tailored with regard to each other. Ions in the bulk electrolyte move in the solution phase under the influence of the Coulombic forces and thermal motion but are not contributing to the double-layer formation.
Step 4 - Charged
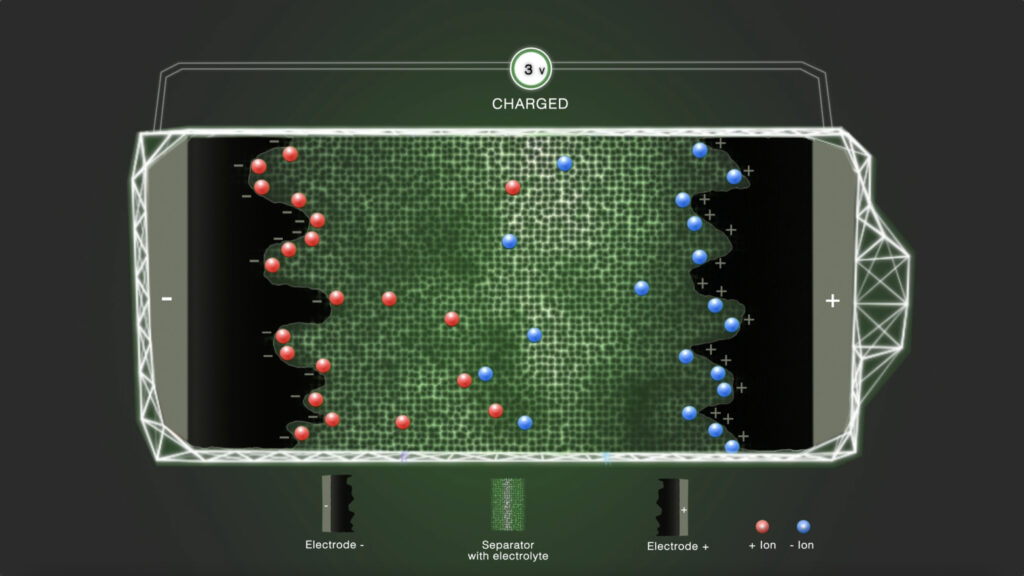
In the charged state, a standard distributed residue of positively and negatively charged ions remains in the electrolyte.
Step 4 – Discharged
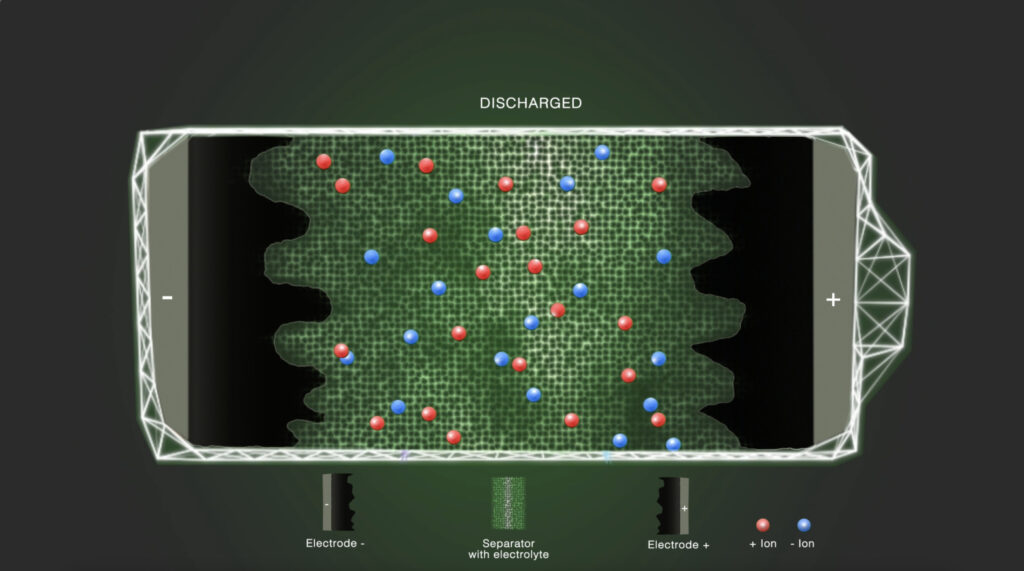
During discharge, the cell voltage decreases, and the attracted ions are released back to the bulk electrolyte thus leading to a simultaneous diminution of the charge layers in the solution and at the electrode surface.
