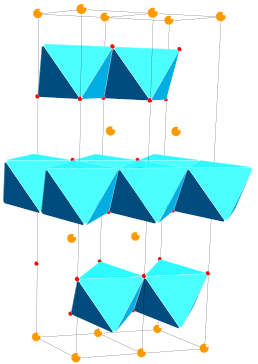
Layered oxides with the general formula LiMO2 (M = Mn, Co, Ni, etc.) crystallize in an α-NaFeO2 structure and are described by the space group (‘O3’ phase), which is based on a cubic close-packed network of oxygen atoms (Wyckoff position 6c) with alternating lithium layers (Wyckoff position 3b) and layers containing the transition metal cations in the octahedral sites (Wyckoff position 3a) (see Figure 2). The diffusion of Li+ ions in the host structure occurs along with a two-dimensional (2D) interstitial space.
LiCoO2 (LCO) is the most commonly used cathode material in commercial LIBs for portable consumer electronics. Even though LCO has a theoretical specific capacity of 274 mAh g−1, it can only deliver ≈50% of this value (≈140 mAh g−1) because of structural instabilities when more than half of the active Li+ ions is extracted from the structure. Due to the overlap of the Co3+/4+ t2g band with the O2− 2p band a deep charge of the electrode will result in oxygen loss from the host structure. The presence of toxic and expensive Co impedes the use of LCO in large scale applications, such as traction batteries and stationary batteries. The research on layered compounds therefore has moved from LCO to its derivatives and solid solutions in which Co is partially or fully substituted by more abundant and environmentally friendly transition metals, such as Ni and Mn.
Currently, LiNiMnCoO2 (NMC) is one of the most important commercial cathode materials, thanks to its high theoretical specific charge of 278 mAh g−1, better thermal and electrochemical stability in the charged state and lower cost compared to LCO. The valence states of the transition metals are mainly divalent nickel (Ni2+), trivalent cobalt (Co3+) and tetravalent manganese (Mn4+). The electrochemically active redox pair is predominantly Ni2+/Ni4+ while the Co3+/Co4+ couple is becoming active only at higher potentials. The electrochemically inactive manganese remains in its original oxidation state (Mn4+) and provides structural and thermal stability at highly delithiated states. Cobalt stabilizes the layered structure and reduces cation mixing (Li/Ni). Due to the similarity in ionic radii of Ni2+ and Li+ ions, Ni/Li site exchange occurs. This phenomenon is termed ‘cation mixing’ in the literature and is associated with phase changes in the host structure, which have a detrimental effect on the cycling performance. However, it is also reported that the higher electrochemical stability of NMC compared to LCO is not only related to their respective density of states, but also to the ‘pillar effect’ of Ni2+ ions in the Li sites, leading to less electrostatic repulsion between the oxygen layers. To address the structural instabilities of highly delithiated cathode materials and to increase thermal stability, doping with heteroelements, such as Al and Mg, has been reported.